We see aging as a reversible epigenetic state
Our work lays at the interphase of aging, epigenetics and cellular reprogramming. Aging is the major risk for most human diseases and one of the most important challenges of the modern world. We aim at increasing our knowledge about why we age with the goal of discovering novel strategies to slow down or reverse the aging process.
About aging…
Aging is the process of becoming older. Aging can be defined as the progressive decline in the capacity of a cell or an organism to resist stress, damage, and disease ultimately leading to death.

What is the cause of aging?

Aging is one of the most complex processes that we can study in nature. For this reason, despite decades of research, the cause of aging remains unknown. Different molecular and cellular processes, known as the hallmarks of aging, have been proposed as drivers of aging including among others mitochondrial dysfunction, telomere shortening, genomic instability and epigenetic dysregulation.
What is the connection between aging and disease?
Aging is the major risk factor for most human diseases including cardiovascular disease, cancer and neurodegenerative disorders such as Alzheimer’s. The older you are, the higher are the chances that you suffer from these diseases and the worst their manifestation will be. For this reason, aging is painful and expensive. Painful for the person that grows older and for their family and friends. Expensive for people and governments due to the high health cost of medical treatment at old age.
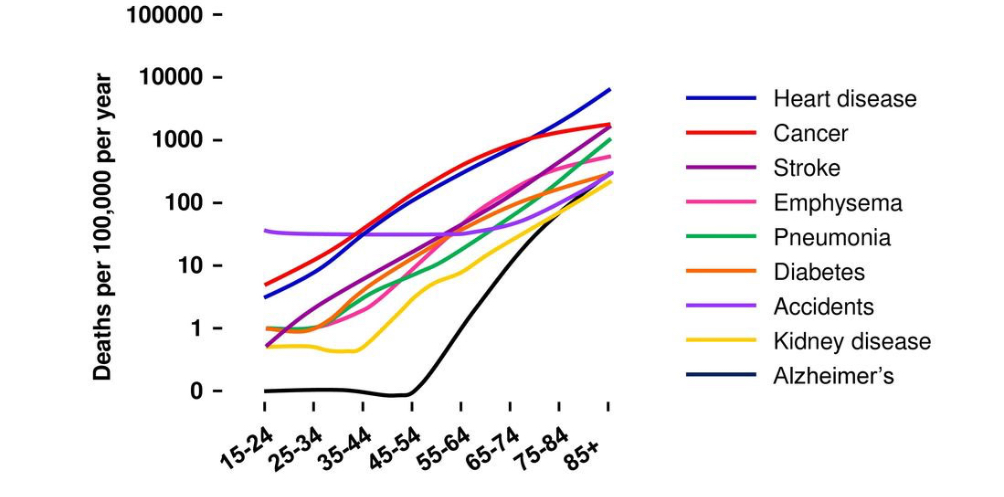
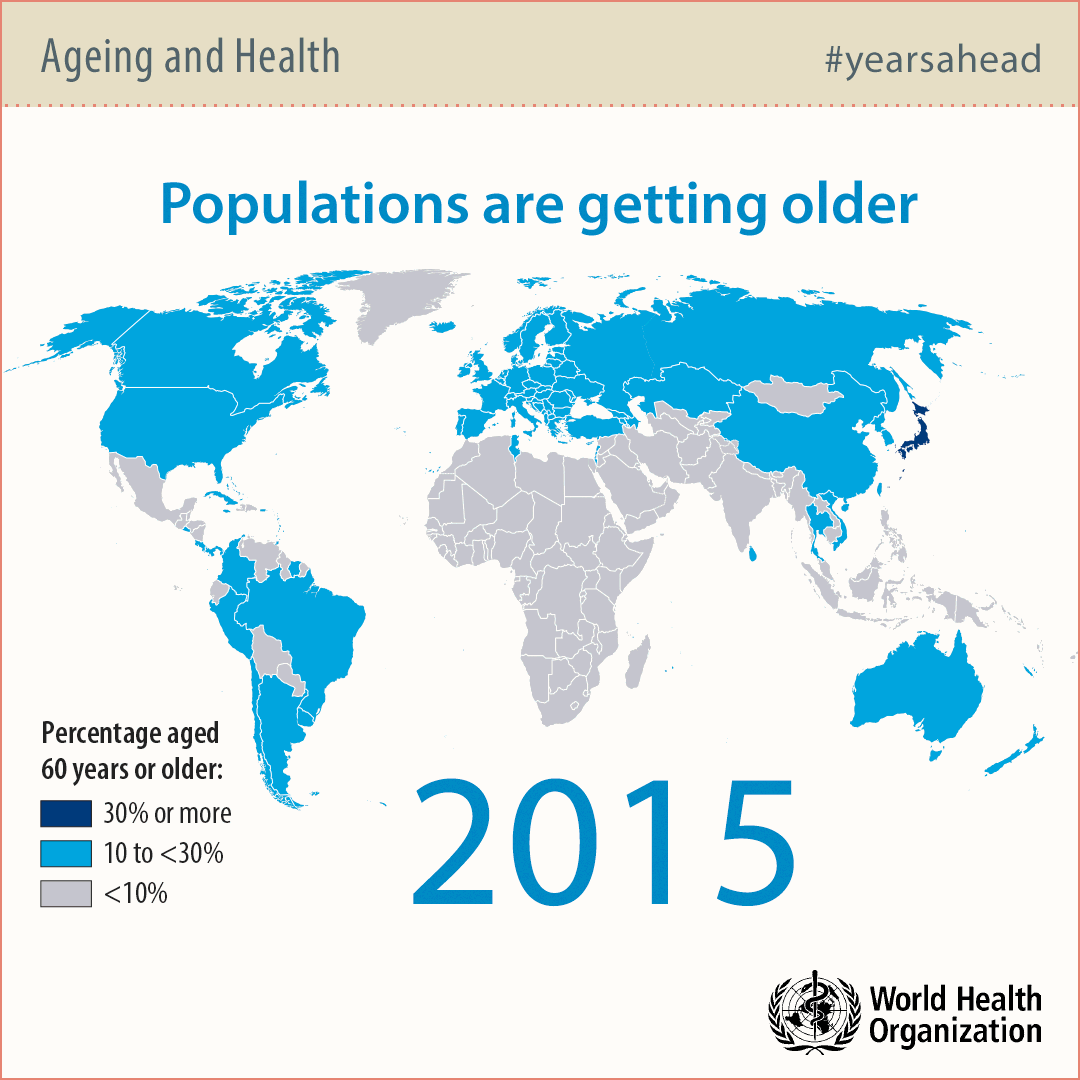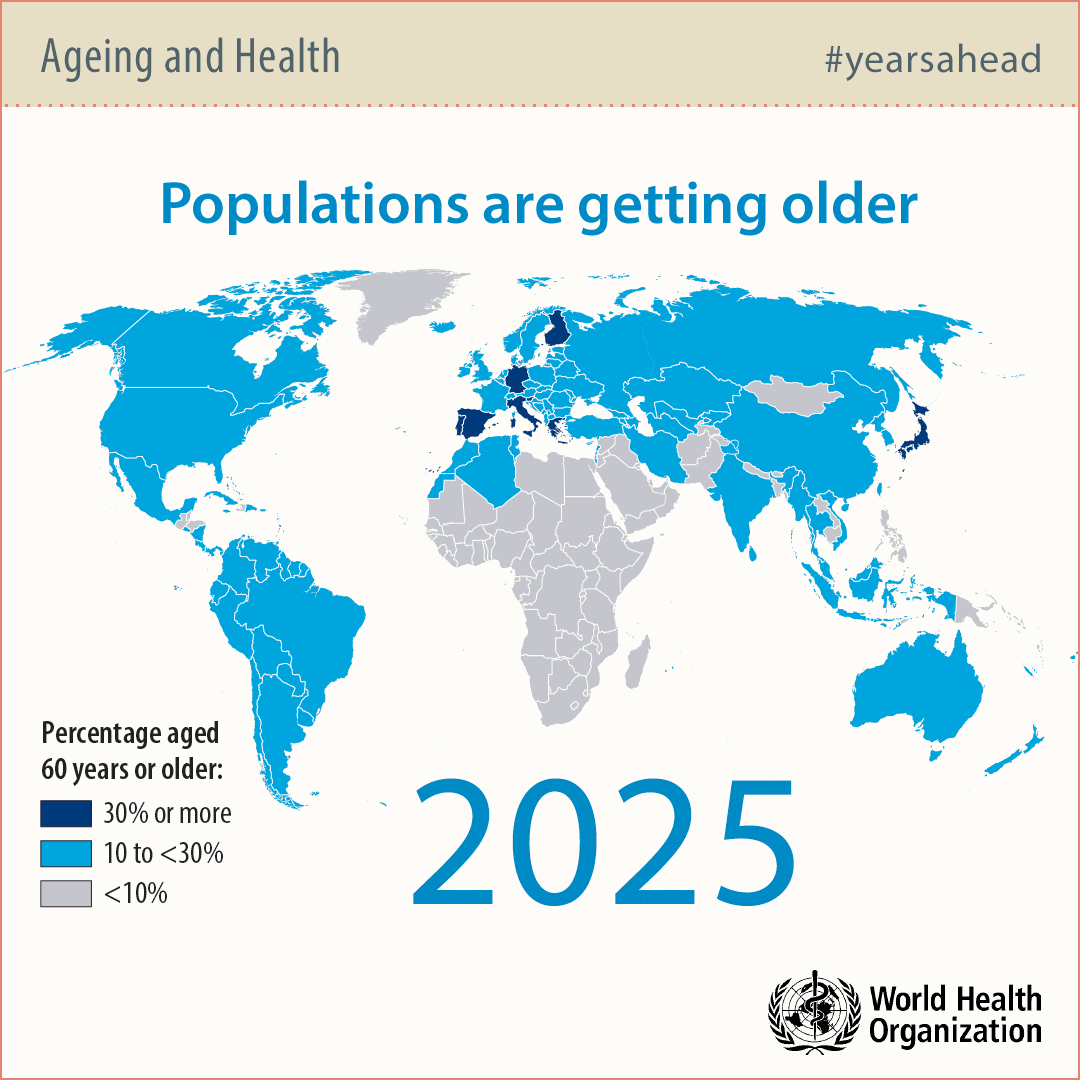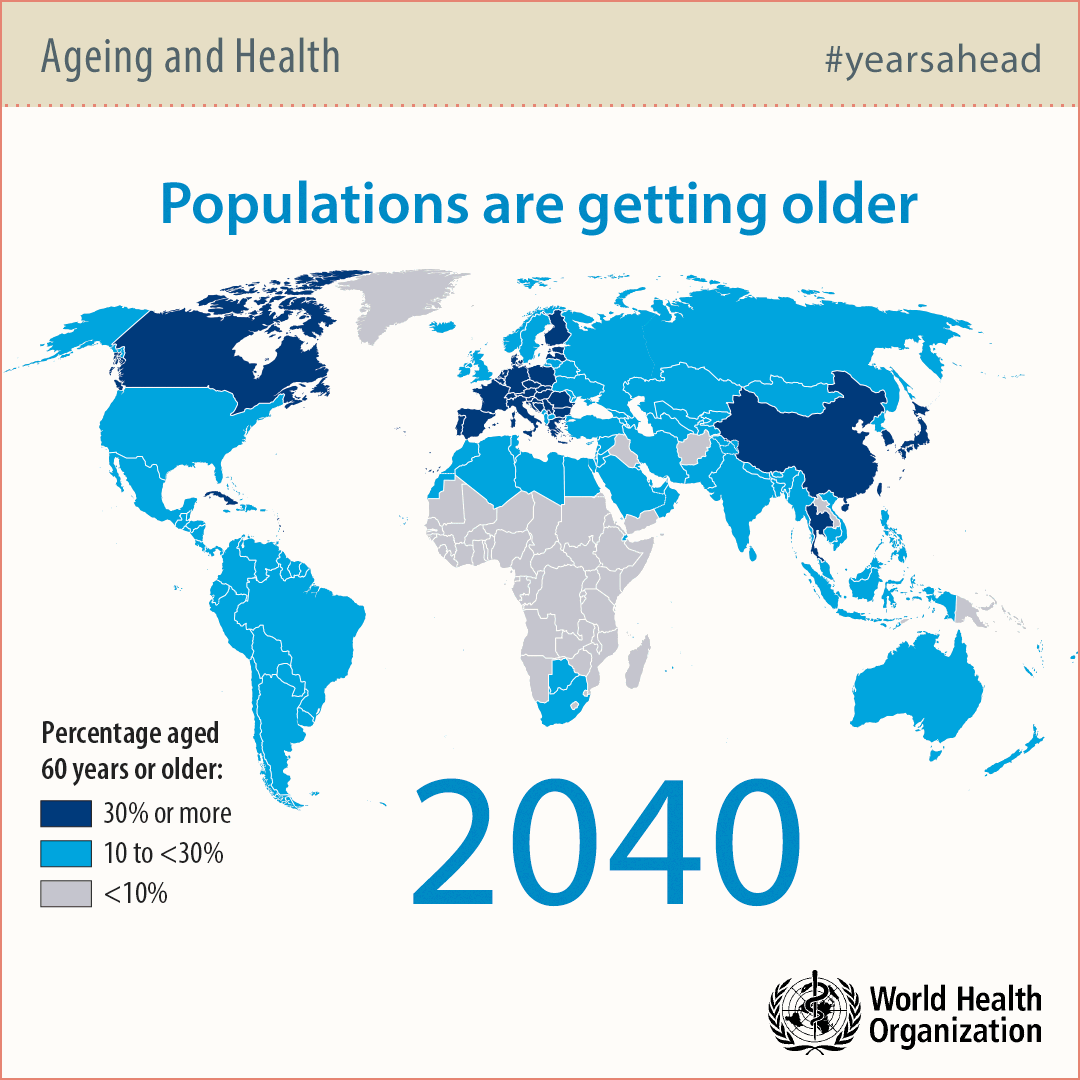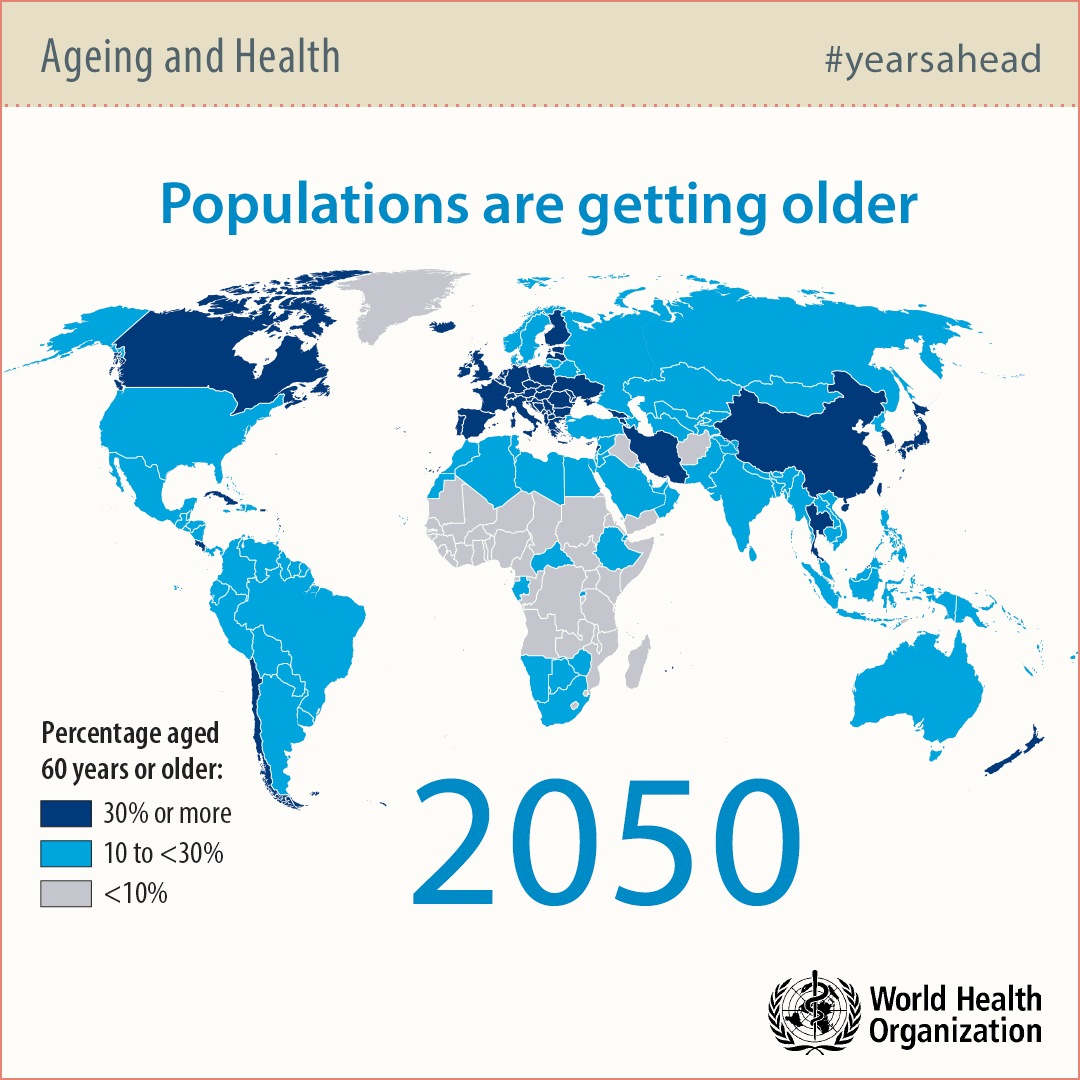
Why so many people are getting old?
Modern societies in developed countries are aging rapidly due to technological advancements in water sanitation, food preparation and medicine. For this reason, the number of people over 65 years of age will double by 2050 significantly exceeding the number of babies.
What will be the effect of slowing or reversing aging on age-associated diseases?
- Average human life
- Extending healthspan
- Extending healthspan and lifespan
Slowing or reversing the process of aging will dramatically reduce the incidence of age-associated disease and increase the period of life that a person stays healthy (healthspan). Anti-aging therapies will therefore extend the healthspan of an individual compressing the period of life that a person is sick. This effect has been observed in model organism where slowing down aging significantly reduces the incidence of disease.
Epigenetic
Epigenetics means “on top of genetics”. In other words, epigenetics can be defined as all the mechanisms that control the expression of a gene that are not related to the sequence of the gene. In other words, epigenetics is everything that is not genetic. Epigenetic mechanisms include binding of transcription factors to the DNA, post-translational modifications of histones (proteins wrapping the DNA), DNA methylation and non-coding RNAs.
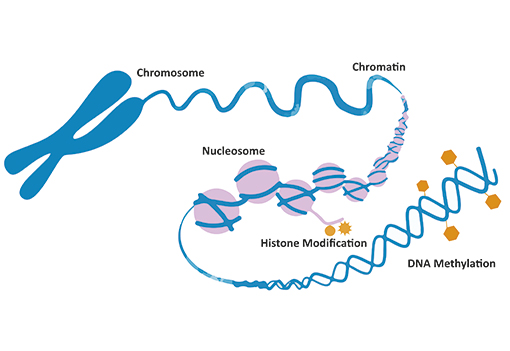
Why is epigenetic regulation important?
Epigenetic regulation of gene expression plays fundamental roles in cellular function. Our body contains more than 200 cell types. Although all the cells share the same genes (DNA), their epigenetic program (epigenome) is different. As an example, a neuron in your brain and a fibroblast in your skin have the same genes (DNA) but they have different epigenetic programs that control the expression of the cell-type specific genes as well as the cell identity and function. These epigenetic programs are established during embryonic development in the womb when embryonic stem cells (ESCs) are transformed into all the cell types in the body in a process known as differentiation. Using an analogy, the cells in our body are like mini-computers that share the same hardware (DNA) but run different softwares (epigenetic programs).
What is the relation between aging and epigenetics?
During aging major epigenetic changes are observed in our cells. While epigenetic regulation is accurate and precise when we are younger, our epigenome becomes progressively deregulated when we are older. As an example, during aging some epigenetic marks increase, some others decrease and others change their position. As a consequence of these changes, the regulation of the genome is less accurate and noisy. Once again, our cells are similar to mini-computers that are very fast when they are new but become slow and corrupt with frequent used as they become older. Nevertheless, although major epigenetic changes are observed and correlate with aging, their role as drivers of the aging process remains still under investigation.
We work on cellular reprogramming
Cellular reprogramming can be defined as the conversion of one cell type into another. Although for many years we thought that different cell types could not be converted into one another, this paradigm was disproved by the work of John Gurdon and Shinya Yamanaka. Their work demonstrated that cells could be reprogrammed back to a pluripotent state capable of generating any cell type by either transplanting their nucleus into an oocyte or by forcing the expression of a set embryonic transcription factors known as the Yamanaka factors (Oct4, Sox2, Klf4 and c-Myc).
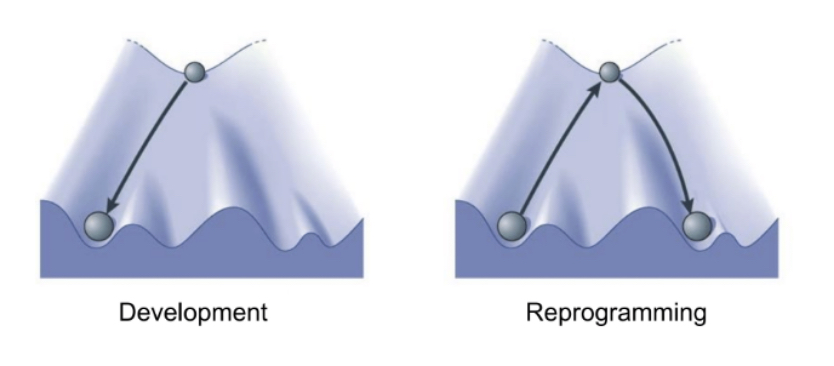
What happens when old cells are reprogrammed to pluripotenty?
Importantly, cellular reprogramming of mouse or human cell isolated from individuals at old age or from patients suffering premature aging syndromes leads to the improvement of age-associated phenotypes. In other words, during cellular reprogramming to pluripotency, old cells are rejuvenated and become younger again. Interestingly, major epigenetic remolding is observed during sexual reproduction and during cloning. In the case of reproduction, oocytes and sperm of advanced age are combined to generate offspring with the aging clock reset to zero. Similar to an old computer, reformatting the software resets the performance of the computer back to new.
Can cellular reprogramming slow down or reverse aging?
Based on the improvement of aging phenotypes observed during reprogramming of cells isolated from old individuals, we decided to test whether cellular reprogramming could slow down or reverse aging in a living organism. In 2016, we demonstrated that cellular reprogramming in vivo could ameliorate age-associated phenotypes and extend the mean and maximal lifespan of a mouse suffering from premature aging by 30%. In addition, we showed that in vivo reprogramming could restore the regenerative capacity of the muscles and the pancreas of old mice back to younger levels (Ocampo A., et al. Cell 2016)